PrismRA For Physicians
PrismRA Delivers Precision Medicine to You and Your Patients with Rheumatoid Arthritis
PrismRA is a blood-based, precision medicine, molecular signature response classifier (MSRC) that predicts inadequate response to TNFi therapy in rheumatoid arthritis.

PrismRA delivers proven, data-driven, precision medicine validated to predict TNFi inadequate response.
Rheumatologists Are Forced to Take a Trial-and-Error Approach to Therapy Selection
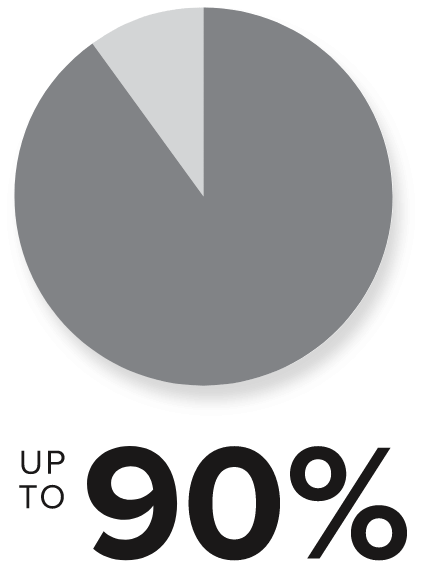
of patients with RA are treated with TNFi therapies as first-line biologic or targeted synthetic DMARD3,4
of patients with RA reach ACR50 at 6 months with b/tsDMARD after failing methotrexate5,6
Knowing the patient’s PrismRA score helps guide decision making to stop the current trial-and-error approach
The PrismRA Molecular Signature Response Classifier Was Developed to Guide Therapy Selection
To develop PrismRA, Scipher started with approximately 7,000 biological features. Using artificial intelligence, we identified and ranked the top 23 features most predictive of inadequate response to TNFi therapies.
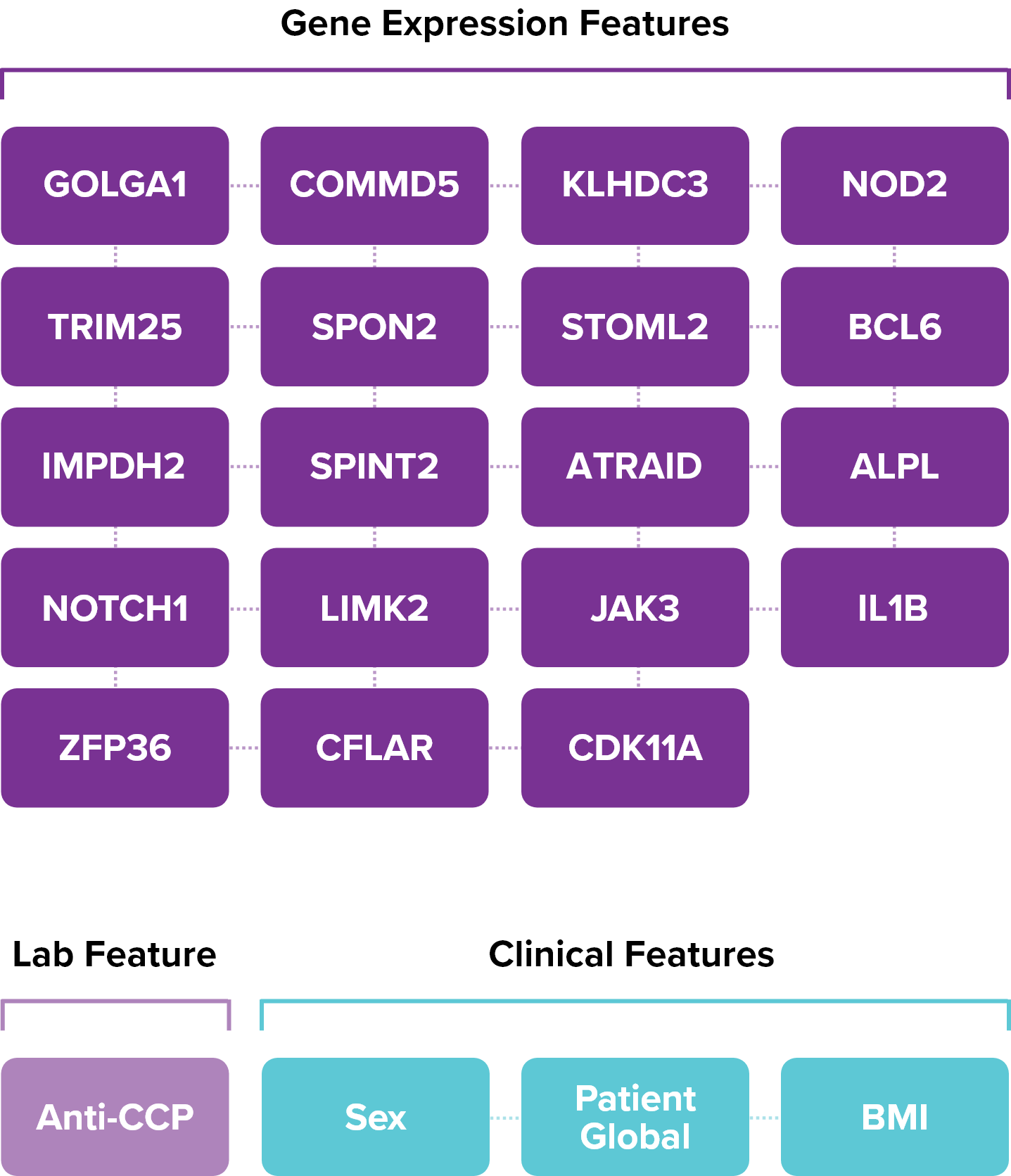
Therapeutic Response Is Predicted by Capturing the Interaction between All 23 Features NOT by Any Single Feature
PrismRA Neural Network Model
An Actionable, Easy to Interpret, Single Page Result to Guide Therapy Selection
- The PrismRA result shows whether a TNFi inadequate response signature is detected.
- The PrismRA score is reported on a scale of 1 to 25. The higher the score, the more likely the patient is to have an inadequate response to TNFi therapies.
- Based on the PrismRA score, PrismRA predicts your patient’s probability of response to TNFi therapy.
PrismRA Provides Unique Insights to Guide Treatment Decisions

>10.6 is a high signal of inadequate response and the patient has a 10% chance of responding to a TNFi

>18.5 is a very high signal of
inadequate response and the patient has a 5% chance of responding to a TNFi

<10.6 indicates that no signal of inadequate response was detected and the patient may be likely to respond to a TNFi*
*A low PrismRA score does not ensure a positive response.
Identify a patient that would benefit from PrismRA
Fill out the single-page test requisition form (TRF) with your patient’s information
Complete a blood draw in your clinic or via our collection services
Package everything in the PrismRA Kit and ship to Scipher with the included prepaid labels
Receive the PrismRA result within 5-7 business days and select therapy based on your patient’s unique biology
Publications and Abstracts
Jeffrey R Curtis, Vibeke Strand, Steven Golombek, et al.
November 3, 2022
Vibeke Strand, Lixia Zhang, Alix Arnaud, et al.
April 23, 2022
Vibeke Strand, Stanley B Cohen, Jeffrey R Curtis, et al.
December 30, 2021
Alex Jones, Sarah Rapisardo, Lixia Zhang, et al.
November 10, 2021
Stanley Cohen, Alvin F Wells, Jeffrey R Curtis, et al.
June 19, 2021
Learn More About PrismRA
Interested in how PrismRA helps guide therapy decisions? Please contact the Scipher Medicine Client Services team.
References
1. Jones A, et al. Expert Rev Mol Diagn. 2021;21(11):1235-1243. 2. Strand V, et al. Expert Rev Mol Diagn. 2022;22(1):101-109. 3. Jin Y, et al. Arthritis Res Ther. 2017;19(1):159.
4. Curtis Jr, et al. Arthritis Care Res (Hoboken). 2014;66(11):1604-11. 5. Incerti, D, Jansen, JP. A Description of the IVI-RA Model v2.0. 2020; last updated January 2020. 6. Curtis JR, et al. Semin Arthritis Rheum. 2010;40(1):2-14.

